ELEVATING TOMORROW’S STANDARD OF CANCER CARE TODAY
10 Million people die from cancer every year. It remains the cause of every sixth death. At Abalytics Oncology, our vision is to redefine the standard for drug development in the 21st century.
How do you approach drug development in the age of artificial intelligence? Where do you choose to focus? At Abalytics Oncology, three simple values drive every action we take:
Develop best-in-class CTLA-4 targeting bispecific antibodies
Develop next-generation multispecific therapies with finely tuned dynamics
Build data model and system integrations to solve immune dysregulation
Abalytics Intelligence
Sequences
Clinical Trials
Structures
Publications
Patents
Biomarkers
Companies
Technologies
Investments
Code
Real-world information, in real-time
Artificial Intelligence generates infinite options, which human intelligence evolved to constrain.
Abalytics Algorithms
Target selection
Antibody design
Lead identification
Translational modeling
Treatment optimization
Resource allocation
Abalytics Pipeline Programs
Bispecific oncology therapies
Bispecific immune cell modulators
Bispecific pMHC targeted TCR mimetics





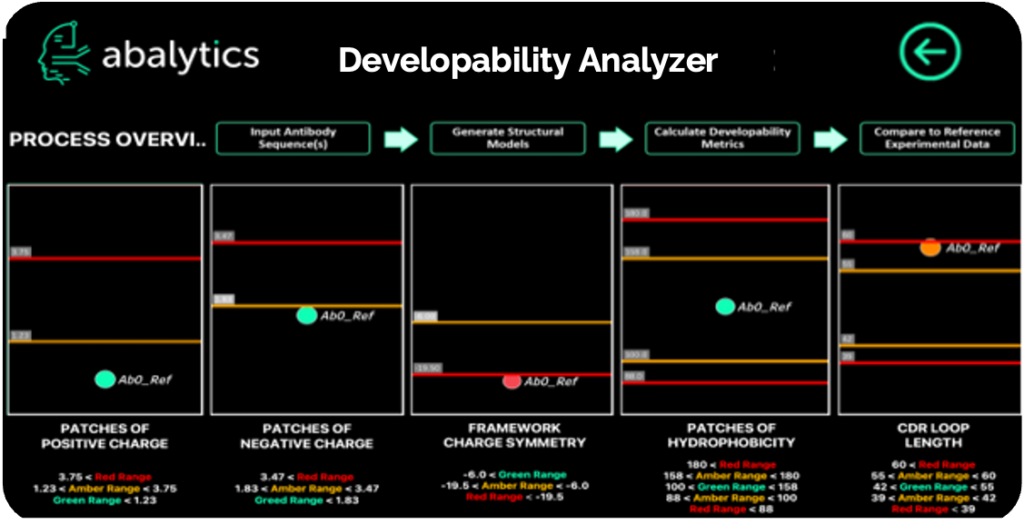

Program
Discovery
IND-Enabling Studies
Status
CTLA-4 targeting multi-specific antibodies with unique dynamics
ABAO5
PD-1 / CTLA-4 / VEGF
Ready for CMC
ABAO4
CTLA-4 / VEGF
Ready for CMC
ABAO3
CTLA-4 / PD-1
Available to Partner
pMHC-targeted bispecific TCR mimetic
ABAO6
pMHC / CD3
R&D
Bispecific targeted degrader
XX / YY
R&D
SEQUENCE
STRUCTURE
PATIENTS SPECIFIC OUTCOMES
DYNAMICS
With only the first prediction problem “solved”, how can we achieve the outcomes we want?

The information age has unlocked new insights into where cancer appears that we can target
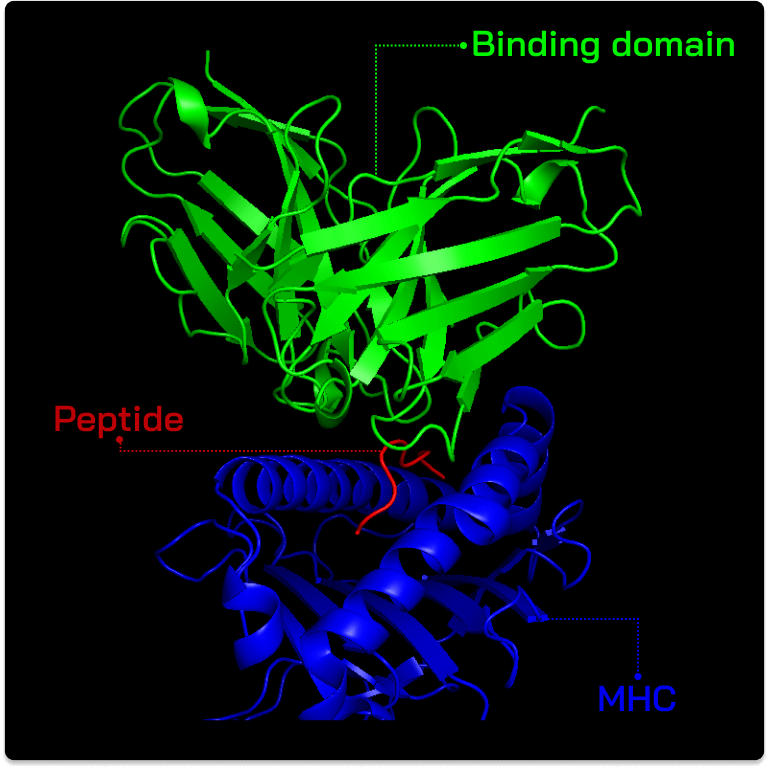
Abalytics computational design offers advantages over existing TCR and TCR mimetic approaches
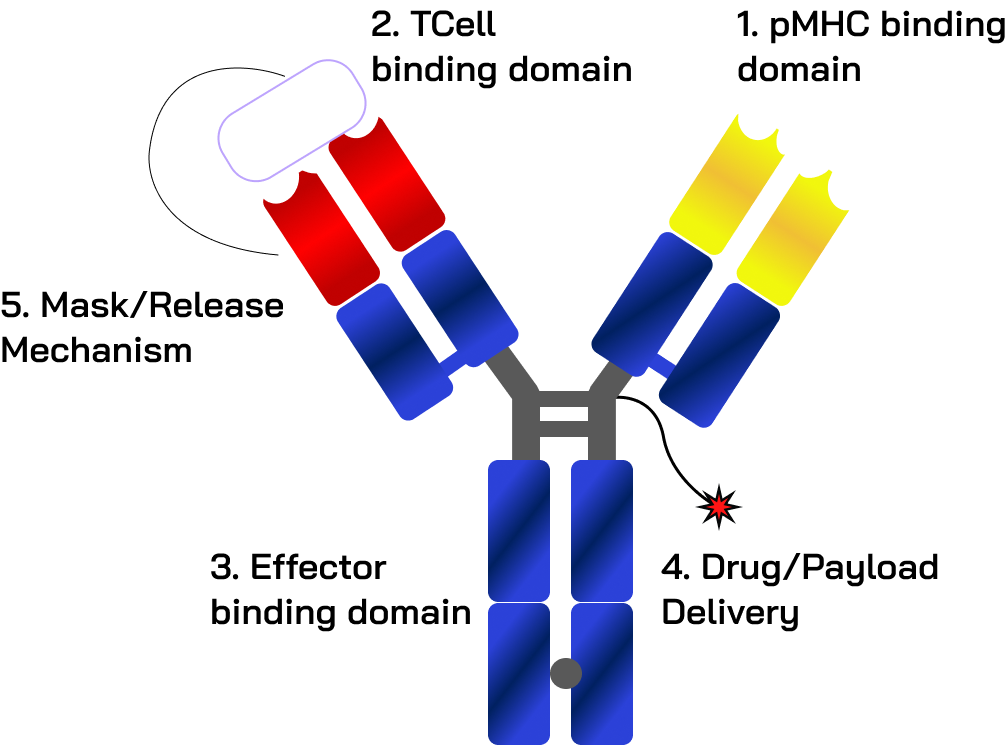
Modular next-generation antibody-based formats offer validated options to optimize safety, efficacy, and developability

Chief Executive Officer

Chief Business Officer

Board Member

Advisor: Scientific and Clinical

Advisor: Scientific and Clinical
Stephen Hodi, MD, is a medical oncologist and principal investigator at Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School. He is director of the Melanoma Center and the Center for Immuno-Oncology at Dana-Farber/Brigham and Women’s Cancer Center. Hodi earned his medical degree at Cornell University Medical College, did his postdoctoral training in internal medicine at the University of Pennsylvania, and completed a medical oncology fellow at Dana-Farber, joining the faculty in 1998. An internationally recognized leader in developing immune therapy and melanoma therapeutics, Hodi is particularly known for the clinical

Advisor: Translational Science and Development

Advisor: Antibody Engineering, Development, and Strategy
Connect with us about reshaping the future of cancer care today.
Shay combines a track record of computational antibody design and development with a decade of experience implementing data analytics systems across diverse healthcare settings. He is credited with the invention of Omoprubart and has executed on over 15 other cutting edge drug development programs, including TY027. Before formalizing a system for computational antibody design in the Sasisekharan Lab at MIT’s Koch Institute for Integrative Cancer Research, Shay developed technology solutions for public and private sector clients exploring practical applications of artificial intelligence. An ex-Deloitte Systems Integration consultant, Shay holds a Bachelor of Science in Chemical and Biomolecular Engineering from Johns Hopkins University.
Manish has a track record of execution in the biopharmaceutical industry. He was the Chief Business Officer of Shinkei Therapeutics, a CNS focused company. He was previously the President and co-founder of Innogenix Pharmaceuticals. At Innogenix, Manish took a brownfield project through FDA facility approval, directed the R&D strategy, in-licensed assets, and grew the revenue to a stable base. He was also the head of business development of Epic Pharma, where he led the sale process of the company to Humanwell Healthcare of China in 2016. Since 2016, he has been actively investing and managing portfolio companies in health care. Manish has overseen R&D, regulatory, commercial, and manufacturing in his career. He began his career in banking and as a trader at a hedge fund. Manish holds a Bachelor of Science in Molecular Biology from Johns Hopkins University, and a Master’s in Financial Engineering from New York University.
Joyson Karakunnel is an experienced medical oncologist and hematologist with over 20 years of experience in drug development both in academia and industry in multiple therapeutic areas and drug modalities. He is an independent consultant for private and public biotechs and VC/PE firms. He serves on the BOD for Primevax and Precision Biologics. He has served as an advisor at the Parker Institute for Cancer immunotherapy. He was CMO at iTeos Therapeutics, Innate Pharma, Tizona Therapeutics and was part of the founding team for Trishula Therapeutics. He has held leadership positions at Arcus Biosciences and Medimmune/Astrazeneca where he was involved in the approval for Imfinzi and Imjudo in monotherapy and combination settings. Prior to joining the pharmaceutical industry, he was an investigator in clinical trials at the NCI and team leader for the hematologic group at Walter Reed National Military Medical Center. He was an Associate Professor at the Uniformed Services University of the Health Sciences and medical reviewer at the FDA. He did his fellowship training in hematology and oncology at the National Cancer Institute and residency training at Overlook Hospital/UMDNJ. He was elected as a Fellow in the American College of Physicians (FACP). He completed his medical training at Annamalai University in India. He holds a Master’s of Pharmacology from the University of Maryland and is a member of Phi Beta Kappa. he holds an MBA from the Kelley School of Business.
Dr. Toni K. Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute, co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. He is the Medical Director, International Strategic Initiatives at Dana-Farber and past President of the Dana-Farber Medical Staff (2016-2018). Dr. Choueiri is an elected member of the American Society of Clinical Investigation (ASCI). In addition, he is an Aresty Scholar from the Wharton School of Business at the University of Pennsylvania. Dr. Choueiri is interested in developing novel experimental therapies and biomarkers in GU malignancies as well as interest in liquid biopsies and markers of toxicity of systemic agents. His work has been published in journals such as the NEJM, Nature, Nature Medicine, Science, Journal of American Medical Association (JAMA), JAMA Oncology, The Lancet, Lancet Oncology, and Journal of Clinical Oncology. He lectures frequently throughout the United States and around the world. He has over 800 PubMed-indexed publications and is the lead investigator of multiple national and international phase I-III trials in GU cancers.
Stephen Hodi, MD, is a medical oncologist and principal investigator at Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School. He is director of the Melanoma Center and the Center for Immuno-Oncology at Dana-Farber/Brigham and Women’s Cancer Center. Hodi earned his medical degree at Cornell University Medical College, did his postdoctoral training in internal medicine at the University of Pennsylvania, and completed a medical oncology fellow at Dana-Farber, joining the faculty in 1998. An internationally recognized leader in developing immune therapy and melanoma therapeutics, Hodi is particularly known for the clinical development of checkpoint inhibitors. He led the first human trial of ipilimumab, which blocks the CTLA-4 checkpoint, and later led the phase III registration trial, which was the first study to show a survival advantage for a melanoma drug, and which led to FDA approval of ipilimumab. The dramatic and long-lasting responses seen with this agent provided proof that reactivating the suppressed immune system of cancer patients could be enormously beneficial. Subsequently, Hodi has continued as a key investigator in the clinical development of the second family of checkpoint inhibitors, which block PD-1 and PD-L1. This work has not only changed the paradigm for treating melanoma but has also been applied to improve outcomes in many other malignancies, such as lung and kidney cancer. He has also led pioneering, investigator-initiated trials combining immune checkpoint blockade with cytokines and the first combination of immune checkpoint blockade with anti-angiogenesis agents. Hodi is also known for his work in treating KIT-mutated melanoma.
Bernard A. Fox, PhD, is Chair of the World Immunotherapy Council (WIC) and past President of the Society for Immunotherapy of Cancer (SITC). He is the Harder Family Chair for Cancer Research, Member and Chief of the Laboratory of Molecular and Tumor Immunology, Earle A. Chiles Research Institute (EACRI), Providence Cancer Institute. He is also Founder, President, and Chief Executive Officer of UbiVac, a clinical stage immunotherapy company with disruptive cancer vaccine technology that contains shared canonical and recently described non-canonical alternative cancer neoantigens. Prior to joining the EACRI, Bernie spent 5 years in Dr. Steven A. Rosenberg’s laboratory at the Surgery Branch, NCI, NIH. His undergraduate studies were at the University of Detroit and he received his PhD from Wayne State University. Dr. Fox maintains a faculty appointment at OHSU and membership in the NCI Knight Cancer Institute. Dr. Fox lectures widely, authored more than 200 papers and book chapters, has served or serves as a professor at universities in Germany, The Netherlands, China, and Taiwan, and as a member of review committees for the NIH, FDA, philanthropic, and governmental organizations in the USA, Europe, and Asia. In 2015, he received the Visionary/Legacy Award from SITC. In 2020 Dr. Fox received a SITC Team Science Award and the Melanoma Foundation Onlus Bridge Award, for outstanding and lifelong contributions to melanoma research. In 2022 he received SITC’s Tara Withington Public Service Award.